products
Newsletter
Get the latest news & events from us, Subscribe Now!
Ibuprofen sustained release capsules
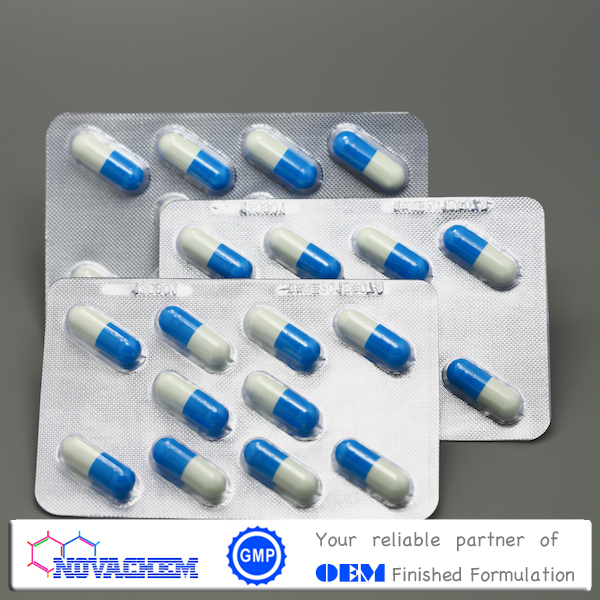
SKU:FFC005
Description:
Description:
CAS No.: 15687-27-1
MF: C13H18O2
EINECS No.: 239-784-6
Place of Origin: Hubei, China (Mainland)
Type: Antipyretic Analgesics and NSAIDs
Grade Standard: Medicine Grade
Brand Name: Novachem
Model Number: 0.3g
Melting point: 77°C
logo: custom-made
Browse Other Products



 print
print close
close